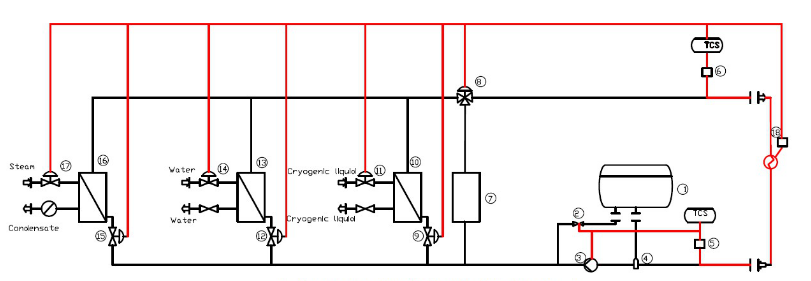
Chemical synthesis process control system temperature control system TCU
Today, let’s talk about our DCS integrated control system (temperature and other control of synthetic process).
Scalability: the system is an open system, which provides standard TCP / P data communication interface protocol, interface software and application software interface. It has good flexibility and scalability, and meets the requirements of continuously improving production scale for measurement and control ability.
Security: the system has a permission mechanism to realize multi-level permission management. The operating users are divided into five different levels, and the operating permissions of users at different levels are also different. The control system is a relatively closed network, and a firewall is configured between the system network and the enterprise LAN or external network, which can effectively prevent viruses and network attacks and create a safe environment for the operation of the system.
Improve the stability between batches of products: according to the process requirements of pharmaceutical batch production, establish an appropriate production model and strengthen the management of pharmaceutical batches. The batch management of pharmaceutical production is realized through the management modules of production plan designation, production plan release and production plan tracking.
Ensure the integrity of production data: through the application of GMP compliant computer management system and automatic control system, realize the complete automatic collection and monitoring of production data, and carry out effective, accurate and complete data collection, analysis and processing through the computer system, so as to avoid the problems such as lag, missing and error of production data easily caused by the traditional manual recording method.
Improve the traceability of production records: the traceability of pharmaceutical production data is GMP The necessary contents of FDA and other pharmaceutical industry certification, the application of pharmaceutical production automation control system and pharmaceutical intelligent production quality information management system software, real-time, complete and accurate comprehensive records of pharmaceutical production data such as batch, product name, time, production equipment number and operators, and the process of reverse retrieval of production data, which can restore the production status of each production link, Enable producers to analyze the causes of product quality defects, and enable producers to clearly understand the actual production status through the data traceability function, so as to provide data basis for improving production. Technological innovation: realize the real-time monitoring and regulation of key process parameters and quality, clarify the key process control parameters and product characteristics, and then optimize the production process and improve the product quality.
Enhance the market competitiveness. Through the application of advanced pharmaceutical equipment and its automatic control technology, the technical level of Danzhu Dingyan product has greatly improved the product quality and output, and improved the market competitiveness of products.
Related recommendations
-
Battery test water cooler common knowledge description
1898Battery test Water-cooled air intake If there is no superheat, there may be a return air with liquid, and even cause a wet stroke liquid shock to damage the compressor. In order to avoid this phenomenon, a certain degree of suction superheat is re...
View details -
Application of Screw Water Chiller
1354The power of screw water chiller is relatively larger than that of scroll chiller. It is mainly used in central air conditioning system or large industrial refrigeration. It can be divided into two categories. 1.Twin-screw refrigeration compressor...
View details -
Matters Needing Attention in Replacing Refrigerant Oil of the Screw Chiller Factory
1553The refrigerant oil in the screw chiller factory needs regular inspection and replacement. We all know that different grades of refrigerant oil cannot be mixed together, otherwise it will cause abnormal or strike conditions of the screw chiller fa...
View details -
What type of temperature control system is required for smear preparation in the pharmaceutical industry?
1402In the pharmaceutical industry, negative pressure drying and smear preparation have specific requirements for temperature control, and usually require precise and adjustable temperature control devices to ensure product quality and process sta...
View details
 LNEYA Industrial Chillers Manufacturer Supplier
LNEYA Industrial Chillers Manufacturer Supplier