How Glycol Concentration Affects Your Glycol Chiller Performance
Glycol is commonly used as antifreeze in cars and as a coolant in industrial chillers. Many people will buy a glycol water mixture of a specific concentration under the advice of experts, but do you know why you should choose that concentration? This article will explain the reasons.
Perhaps before reading this article, you want to explore:
1. Why Is Glycol Used in Cooling Systems?
2. Glycol Chiller vs. Water Chiller: Which Is a Better Choice?
3. Chiller Fluids: Water, Glycol, Oil, and More
4. Guide to Glycol Recycling in Chiller Systems
What is the Concentration of Glycol?
When glycol is used alone, its viscosity is too high and its thermal conductivity is poor. When it is mixed with water, a glycol-water mixture with low viscosity and high heat transfer efficiency can be obtained at the right concentration. Therefore, usually glycol concentration refers to the mixing ratio of glycol to water. For example, 30% glycol means that there are 30 liters of glycol and 70 liters of water in every 100 liters of glycol-water mixture.
How Glycol Concentration Affects Your Glycol Chiller Performance?
Changes in glycol concentration will change its physical properties, which in turn affects the performance of glycol chillers.
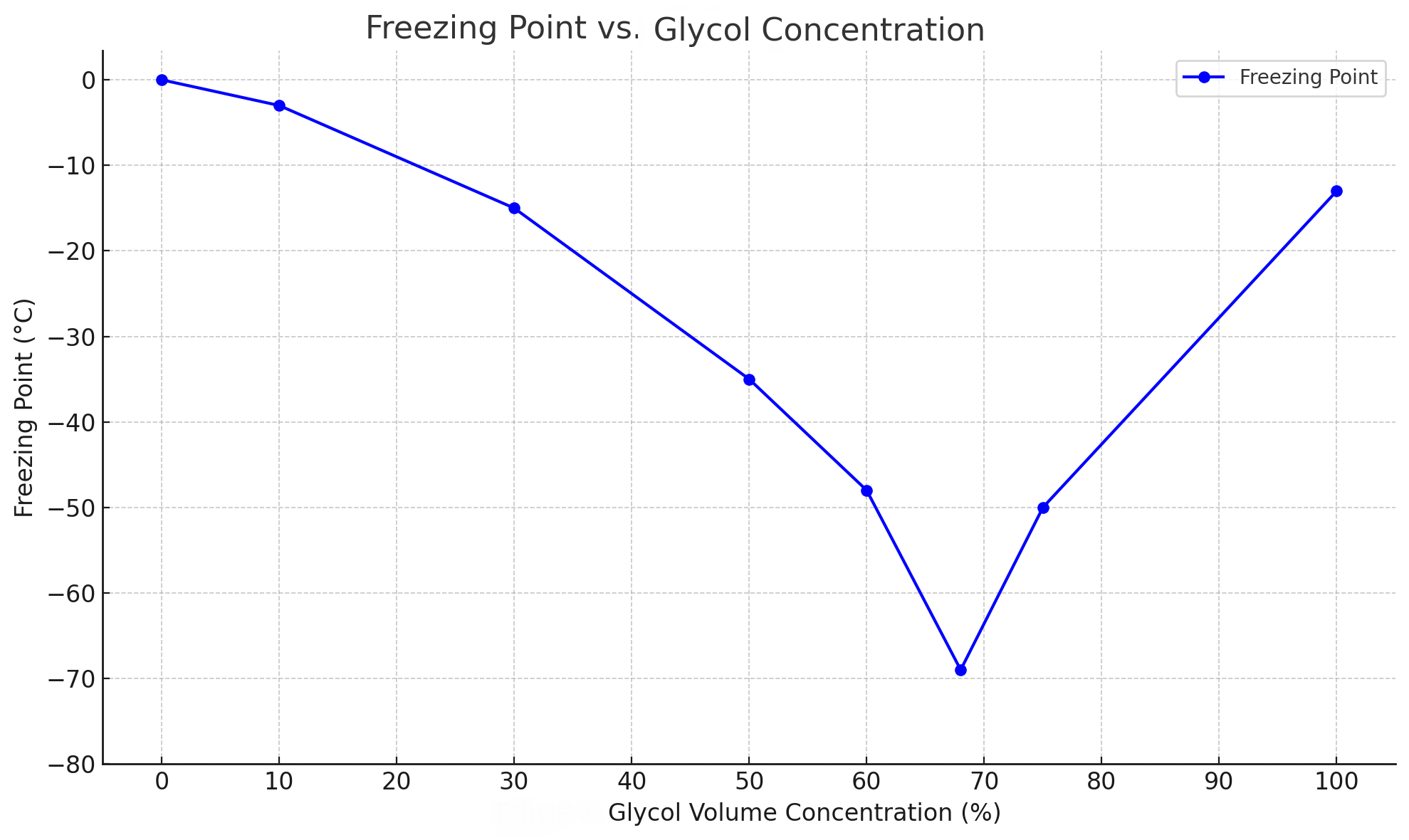
Freezing point
The freezing point is the temperature at which a liquid begins to freeze. For example, the freezing point of pure water is 0°C, which means it freezes at 0°C. If pure water is used as a coolant in a low-temperature chiller, the water in the pipe will freeze when the operating temperature of the equipment reaches below 0°C, thereby damaging the pipe and heat exchanger. However, if glycol is added to water, the freezing point of the mixture will be lowered, and there is no need to worry about freezing even if it is used in a low-temperature chiller system.
The higher the glycol concentration, the more the freezing point drops. However, there is no linear relationship between the two. When the concentration is below 60%, the freezing point of the mixture will decrease as the concentration increases. However, after the concentration is above 60%, the freezing point will decrease slowly and reach the lowest freezing point (about -69°C) at a concentration of 68%. If the concentration continues to increase, the freezing point will rise instead.
When the glycol concentration is too low, the freezing point is too high, and the fluid may freeze in low-temperature chillers. However, when the concentration is too high, the viscosity of the solution will increase, and too slow flow will affect the heat transfer efficiency. Therefore, our experts recommend that the glycol concentration be determined according to the minimum operating temperature of the chiller. If the operating temperature is below -40℃, we do not recommend using glycol as a coolant to ensure the normal operation of the system.
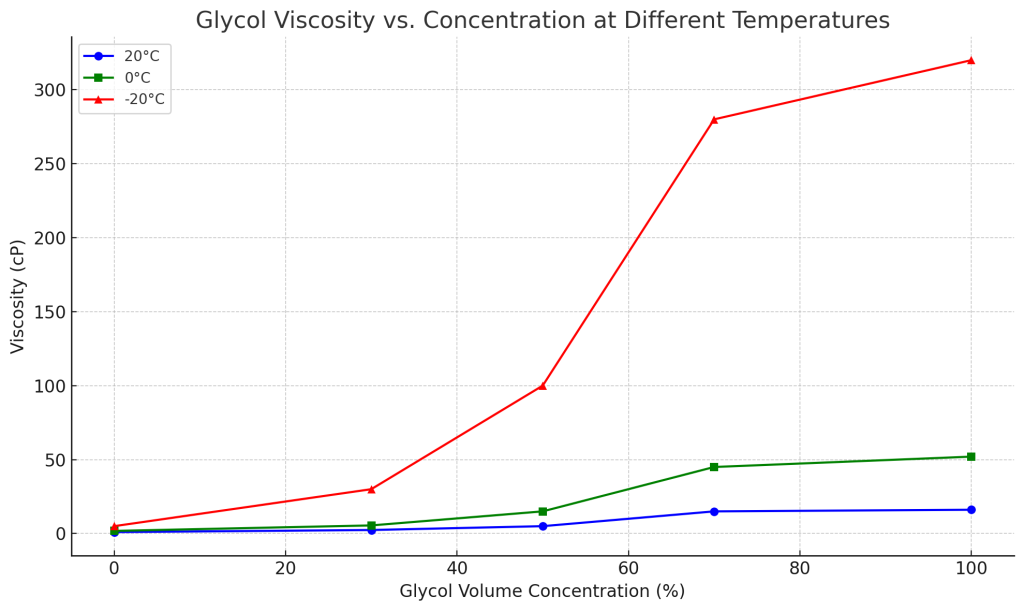
Viscosity
The change in concentration will affect the viscosity of the solution. When the glycol concentration increases, the liquid will become more viscous and stick to the pipe wall and be difficult to flow. Moreover, after the temperature drops, the viscosity of the solution will increase again. For example, the viscosity of 50% glycol at 20℃ is 5cP, but at -20℃, the concentration rises to more than 100cP.
When pumping liquids with higher viscosity, the pump consumes more energy, increasing the energy consumption of the entire cooling system. When customizing glycol chiller systems for customers, we will comprehensively consider antifreeze requirements and fluidity, and use a glycol-water mixture with a concentration of 30%~50% according to the actual operating temperature.
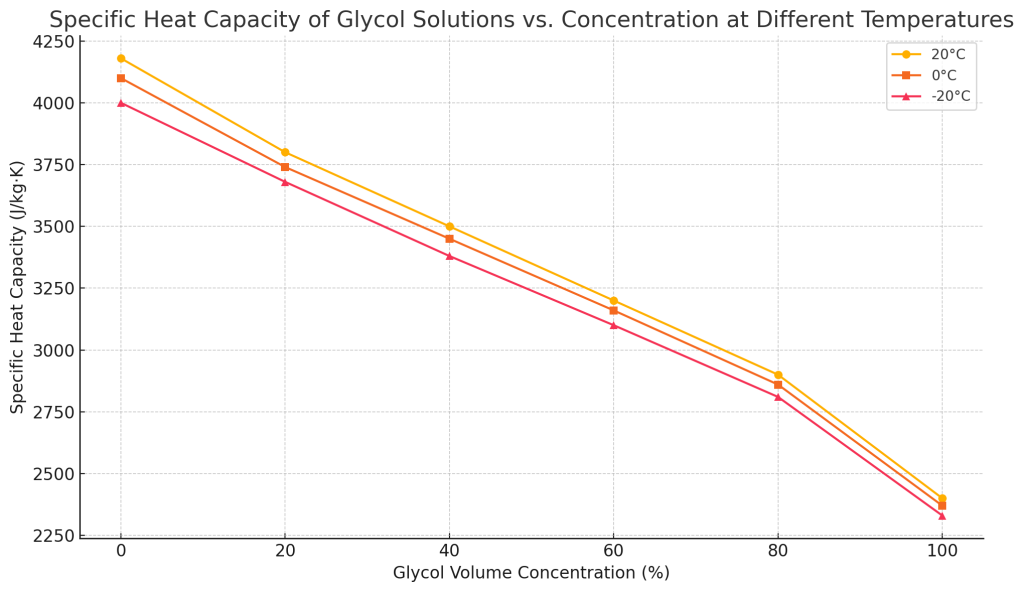
Specific heat capacity
The specific heat capacity determines the speed of the temperature change of the coolant when absorbing and releasing heat. The higher the specific heat capacity, the higher the heat transfer efficiency of the liquid. As the concentration of glycol increases, its specific heat capacity decreases, and less heat is removed in the same flow rate and cooling time. Therefore, if you only pursue antifreeze and ignore the specific heat capacity of the solution, it will inevitably affect the cooling efficiency of the industrial chiller.
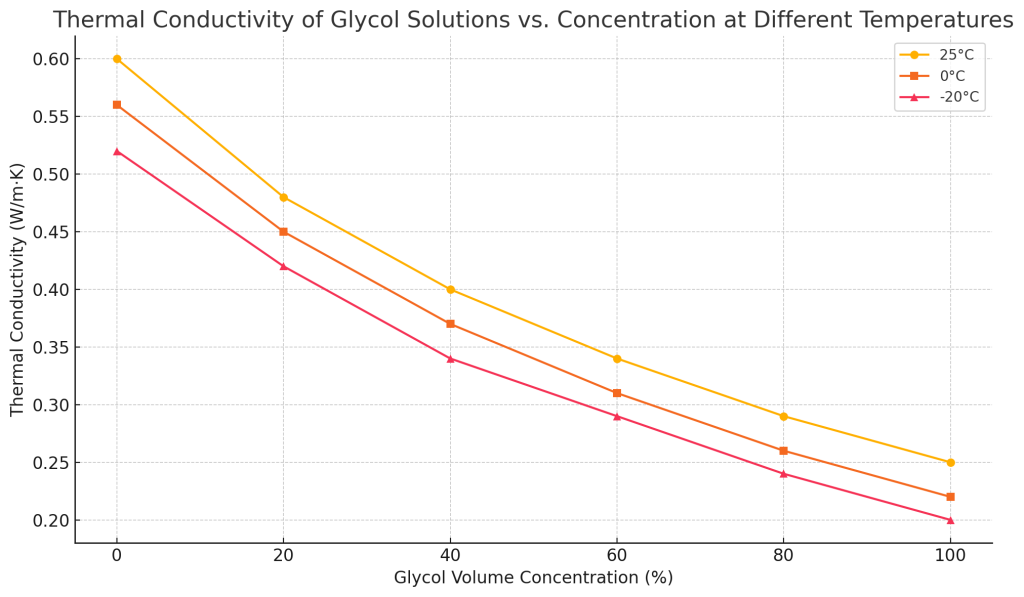
Thermal conductivity
Thermal conductivity indicates the ability of a liquid to transfer heat. As the concentration of glycol increases, its thermal conductivity decreases, that is, the heat transfer efficiency of the liquid decreases. Under the premise of not affecting the operating temperature, using a lower concentration of glycol can ensure better thermal conductivity.
Conclusion
Looking for a glycol chiller? Or still have questions about glycol concentration? LNEYA team is happy to provide you with customized cooling solutions and technical support.
Related chillers
CONTACT US
TEL:
EMAIL:
WeChat & WhatsApp:

Wechat QR

Have a question or need a quote? Fill out the form below, and our team will get back to you within 24 hours.
 LNEYA Industrial Chillers Manufacturer Supplier
LNEYA Industrial Chillers Manufacturer Supplier